OAN Shannon Kelland
UPDATED 5:57 PM PT – Thursday, December 29, 2022
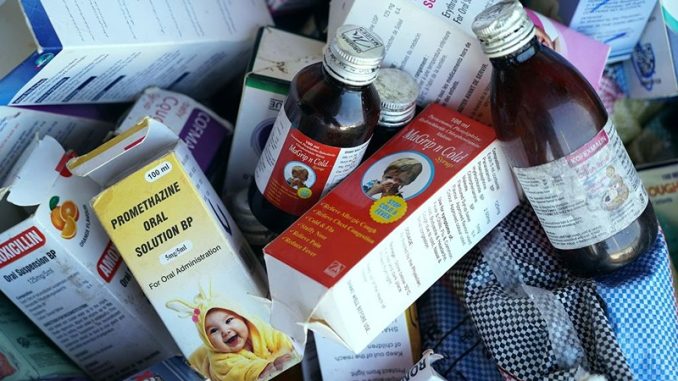
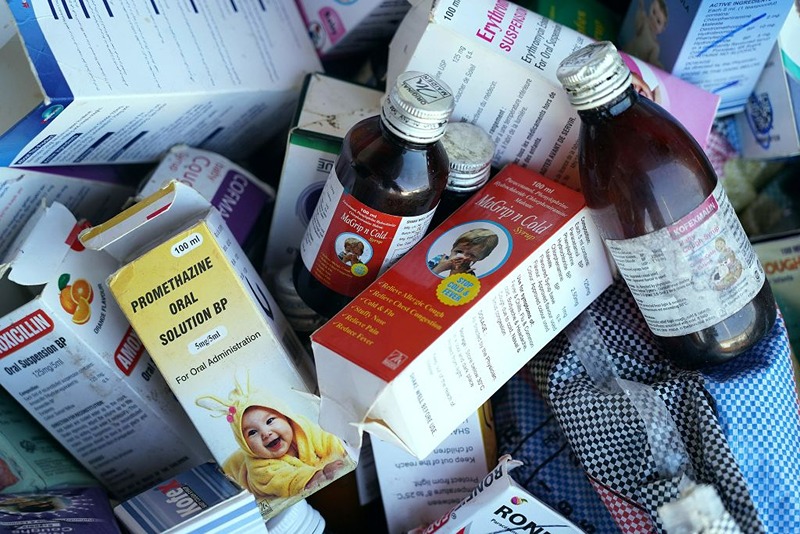
18 kids are dead after consuming Indian-manufactured syrup.
Uzbekistan’s government claims that 18 children died after ingesting a cough syrup made by a manufacturer in India, Indian police services are investigating the matter.
The Uzbek Health Ministry released a statement on Tuesday, where they said a preliminary study of the Doc-1 Max syrup produced by a small pharmaceutical manufacturer Marion Biotech Ltd., based in the Delhi. It was concluded that it contained toxic ethylene glycol, which was responsible for the fatalities. The products have been withdrawn from shelves and not available for sale any longer.
Uzbekistan has also launched legal action against Marion Biotech’s local representatives.
This is not the only manufacturer.
Back in October, the World Health Organization (WHO), issued a warning after four cold-and-cough syrups made by another little-known New Delhi-based firm, Maiden Pharmaceuticals Ltd. The medications were allegedly linked to acute kidney injuries and dozens of infant deaths in Gambia. They were tainted with “unacceptable amounts” of diethylene glycol and ethylene glycol. Gambian lawmakers called for the drug maker to be sued.
Earlier this month, the Central Drugs Standard Control Organization, India’s regulator rejected the organization’s findings. WHO said that samples taken from Maiden’s plant did not find any toxic substances. The company denied any wrong-doing.
CDSCO Director General V.G. Somani claimed that the WHO’s accusation caused “irreparable damage” to the reputation of the pharmaceutical industry.
Arindam Bagchi, a spokesman for India’s Ministry of External Affairs addressed the claims of Uzbekistan.
“Uzbek authorities have not formally taken up the matter with us. Nevertheless, our embassy has contacted the Uzbek side and is seeking for the details of their own investigation,” Bagchi said.

Be the first to comment